Nov 15, 2011
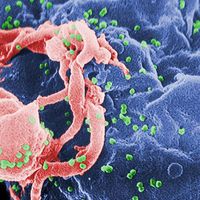
In the early 1980s, epidemiologists were racing to understand a mysterious disease that was killing young men in California. As we now know, that disease was AIDS. And it soon grew into one of the biggest global pandemics in human history. But back in 1984, no one knew what it was, or how it was spreading. So the CDC commissioned a study (abstract here) to look at whether it might be sexually transmitted. And the results were startling--the data seemed to point to a figure at the center of the outbreak from which all the other cases radiated. A few years later, Randy Shilts published a formative book on AIDS called And the Band Played On, which, along with documenting the early history of AIDS in the US, revealed the name of the man at the center of that CDC study: Gaetan Dugas. Dugas was soon dubbed Patient Zero, and labeled by the media as the cause of the AIDS epidemic. But as Carl Zimmer and David Quammen explain, Dugas was absolutely not Patient Zero. Not by a long shot. Michael Worobey and Beatrice Hahn help us search for a much earlier Patient Zero, by taking us to Africa, and turning back the clock on a series of virus mutations and pinpointing one fateful moment of cross-species spillover in a jungle in Cameroon. And virus hunter Nathan Wolfe takes us back even farther, to an intracellular instant that created a chimp Patient Zero hundreds of thousands of years ago.
You can listen to Carl Zimmer's full conversation with Michael Worobey on the podcast "Meet the Scientist" here.
Read More:
Nathan Wolfe, The Viral Storm
Randy Shilts, And the Band Played On